High pH Soils in Wisconsin: What you need to know
What is Target or Ideal Soil pH?
Where and Why High pH Occurs in Wisconsin
How Does High Soil pH Affect Crops?
When we talk about tough soil conditions in Wisconsin, high pH is not the first thing that comes to mind. But in certain counties and field conditions, it can be a persistent barrier to crop productivity. Understanding where high pH occurs, how it affects crop performance, and what you can do about it is essential for keeping yields strong and costs in check.
What is soil pH?
Soil pH is the amount of hydrogen (H+) and hydroxyl (OH–) ions. Soil pH results are on a scale from 0-14.
- A pH below 7.0 indicates acidic soil.
- A pH of 7.0 is a neutral soil.
- A pH above 7.0 indicates alkaline soil.
Look at your recent soil report as soil pH is the cornerstone of a good crop fertility program. More on soil pH.
What is Target or Ideal Soil pH?
Each crop has a target or ideal pH range at which it grows best. Most Wisconsin row crops prefer slightly acidic soil with soil pH between 6.0 and 6.8. While certain crops can adapt to higher or lower pH conditions, other crops are much more sensitive to soil pH. Here is the target pH ranges for common Wisconsin row crops: (See A2809 table 4.2 for full list)
- Corn: pH 6.0–6.8
- Soybeans: pH 6.3–6.8
- Alfalfa: pH 6.8–7.2
- Wheat: pH 6.0–7.0
When Is Soil pH Too High?
Once soil pH reaches 7.5, you may begin to see problems based on the crops you grow. Growing crops with a low target pH is not recommended if your soil pH is 7.5 or higher. The good news is that most Wisconsin soils with a soil pH of less than 7.5 can be amended to lower the pH to the desired level. See below on how to manage high pH.
Where and Why High pH Occurs in Wisconsin
High soil pH can show up in specific situations across Wisconsin:
Calcareous Soils
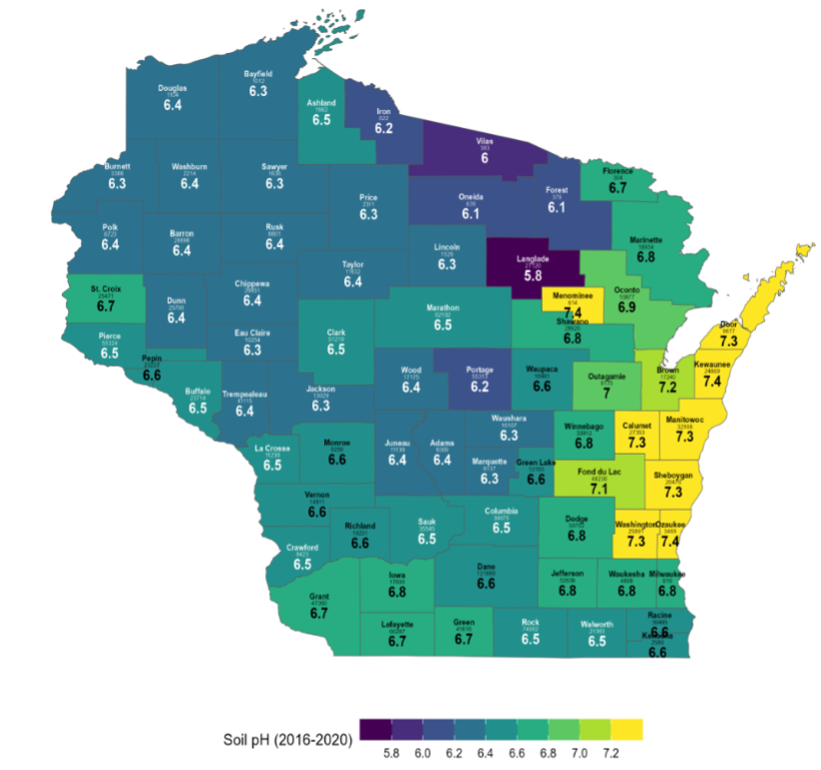
Calcareous soils are characterized by the presence of calcium carbonate (CaCO3) in the soil parent material (limestone bedrock) and an accumulation of free CaCO3 in the soil. The pH of calcareous soils is usually above 7, where the “free lime” (CaCO3) buffers pH and makes it difficult to lower the soil pH. See Figure 1; the counties shaded in yellow have high pH.
Figure 1. 2016-2020 Average Soil pH by County, UW Soil and Forage Lab
Cleaner Air
Acid rain can be defined as the combination of dry and wet deposition from the atmosphere of nitric and sulfuric acid. Deposition of sulfur and nitrogen for a long time regulated the magnitude of soil pH change. However, the amount of “free” sulfur deposited on crop fields from acid rain has decreased dramatically over the past several decades, and pH is creeping up.
According to the Environmental Protection Agency (EPA) the reduction in total sulfur deposition (wet plus dry sources) in the Midwest U.S. was 81% from 2000–2002 to 2020–2022, Progress Report – Atmospheric Deposition | US EPA . The ongoing decline in sulfur atmospheric depositions has had a direct effect on sulfur levels in soils across the Midwest. As recently as 2000, the eastern Corn Belt received 15 lbs of sulfate sulfur per acre per year in deposition. Today the Eastern Corn Belt receives less than 4 or 5 lbs of sulfur per acre per year. Read more Correcting Sulfur Shortages in Midwest Soils | Science Societies.
Poor Drainage
Wet areas, especially low-lying depressions or compacted headlands, can accumulate salts, cations, and bicarbonates (dissolved minerals). When the water finally drains or evaporates, soil pH increases over time. A well-drained soil would allow water to flow and leach excess salts out of the rooting zone, preventing the buildup of the salts from affecting surface pH.
Manure and Compost
Manure and other compost/organic materials can sometimes raise soil pH. In neutral to high pH soils, these materials typically have less impact on pH than in acidic soils. Both manure and compost contain base forming cations such as calcium, magnesium, potassium, and sodium. These base-forming cations can replace hydrogen (H+), hydronium (H3O+), and Aluminum (Al3+) on soil cation exchange sites, which can lead to neutralizing soil acidity. Manure from animals fed high calcium diets (poultry and dairy), may have an inherently higher pH. The decomposition of compost and manure also releases bicarbonates and other alkaline compounds that contribute to pH increases. The overall effect depends on the type of manure or compost, application rate, initial soil pH, and buffering capacity of the soil.
Nitrate-Based Fertilizer Use
Nitrogen fertilizers can raise or lower soil pH depending on the form of nitrogen. As plants uptake nitrate nitrogen, they release hydroxide (OH–) ions which can slightly increase soil pH. The effect is much less than the overall acidifying effect of ammonium-based fertilizers which lower soil pH with intensive use.
Highly Eroded Areas
When topsoil is moved by erosion leaving behind the subsoil to grow crops. Subsoil can have a much different pH than the areas of the field with topsoil.
How Does High Soil pH Affect Crops?
High soil pH can quietly limit crop growth by disrupting nutrient availability. Iron (Fe), boron (B), zinc (Zn), manganese (Mn), and copper (Cu) become limited, leading to deficiencies even if total levels (based on a soil test) are sufficient.
According to UW–Madison Extension publication A3554, the concentration of iron in the soil solution decreases sharply as the soil pH increases, with a minimum pH 7.4-8.5. It is in this pH range that most cases of iron deficiency occur.
Phosphorus (P) becomes fixed as calcium phosphate, making it less available to plants.
Combined factors reduce yield in certain crops, especially corn, once soil pH reaches 7.5 (see Table 1 below). However, overall extreme low soil pH is more impactful on crop yield.
Table 1. Crop Yields Relative to pH, USDA-NRCS
| Soil pH | |||||
|---|---|---|---|---|---|
| Crop | 4.7 | 5.0 | 5.7 | 6.8 | 7.5 |
| Relative Yield | |||||
| Corn | 34 | 73 | 83 | 100 | 85 |
| Wheat | 68 | 78 | 89 | 100 | 99 |
| Soybeans | 65 | 79 | 80 | 100 | 93 |
| Oats | 77 | 93 | 99 | 98 | 100 |
| Barley | 0 | 23 | 80 | 100 | 93 |
| Alfalfa | 2 | 9 | 42 | 100 | 100 |
| Timothy (grass) | 31 | 47 | 66 | 100 | 95 |
How Does High pH Affect Fertilizer and Herbicide Efficacy?
Read your product labels. Herbicide label restrictions limit the use of common herbicides on high pH soils. Sulfonylurea herbicides, used in corn and soybeans, are more persistent in high pH soils, increasing the risk of crop injury and carryover issues. Phosphorus fertilizers are less efficient at high soil pH. This is because much of the applied phosphorus becomes quickly unavailable. Ammonium- based fertilizers may convert more rapidly to nitrate, increasing the risk of leaching. It is also important to know the pH of the water used in the mix tank. A pH of 7.0 is ideal.
How to Manage High Soil pH
Managing high pH is not about making drastic changes. It is about adopting practical, field specific practices that keep nutrients available and crops thriving. If soil pH is 7.5 or greater, growing crops that have a low target pH is not recommended; it would cost too much to alter the pH. A soil pH of 7.4 or less can be amended to lower the pH to the desired level here are some methods how:
Zone or Grid Soil Sampling, Mapping, and Management
Use 2.5-acre grid soil sampling to identify high-pH areas. Then manage the pH zones differently with variable rate application.
Apply a Sulfur Source
Elemental sulfur will acidify the root zone and lower soil pH just as you would add lime to acid soils to raise pH. When elemental sulfur is applied to the soil, it combines oxygen and water to form sulfuric acid. Microbes are the driving force of the oxidation of sulfur, and it may take from 3 to 6 weeks or longer, depending on the soil conditions. The finer the sulfur is ground, the more rapid the conversion to sulfate and dilute sulfuric acid. The more calcium carbonate present (like in calcareous soils), the more buffered the soil is, and the longer it takes to adjust soil pH. See Table 5.3 in A2809.
- While amending soil pH retest the soil each year.
- Be cautious with rates; repeated heavy applications may affect soil biology. Applying more than 20 lbs. sulfur/1000 sq ft per year is not recommended.
- Nitrification is when soil bacteria convert ammonium to nitrate. On calcareous soils continually high rates of nitrogen fertilizer has an acidifying effect over time.
- Aluminum sulfate can also be used to lower soil pH. Its effect is immediate, but the cost is higher than elemental sulfur. The amount of aluminum sulfate needed to lower soil pH is six times the amount of elemental sulfur for the same pH adjustment.
- Note: Products containing sulfate sulfur (gypsum, potassium sulfate) are not effective at lowering pH.
Starter Fertilizer
Starter fertilizer placed near the seed helps young roots access key nutrients before the soil can tie them up in high pH soil.
- Use orthophosphate-based phosphorus sources for better availability.
- Consider adding micronutrients (Zn, Fe, Mn) to starter blends particularly in corn on high pH soils.
- Banding fertilizer is often more effective than broadcasting in high pH soils.
Select Crop Genetics for Tolerance
Some crop genetics are better adapted to high pH conditions or are more efficient at nutrient uptake. An example includes selecting soybean varieties with good iron chlorosis tolerance.
- Work with seed dealers to identify varieties that perform well in calcareous or high pH.
- Keep records of hybrid performance on known high pH spots to inform future planting decisions.
Prevent erosion with a conservation professional and correct drainage with a tiling expert.
Key Takeaways
High soil pH does not have to derail your yield goals. With smart fertility planning, informed seed choices, and targeted nutrient management, Wisconsin farmers can manage high pH zones effectively. Like most soil challenges, the key is understanding your field’s variability and then matching your inputs to what is happening in the root zone. If you are adjusting pH, use soil tests to monitor the changes each year until you reach your desired pH level. Need help interpreting your soil tests or adjusting your fertilizer plan? Talk to your local agronomist or Extension specialist. High pH may be tough, but it is not unbeatable.
Updated: July 11, 2025
Reviewed by: Dan H. Smith, Jordan Kampa, Dan Marzu, and Chris Bandura



