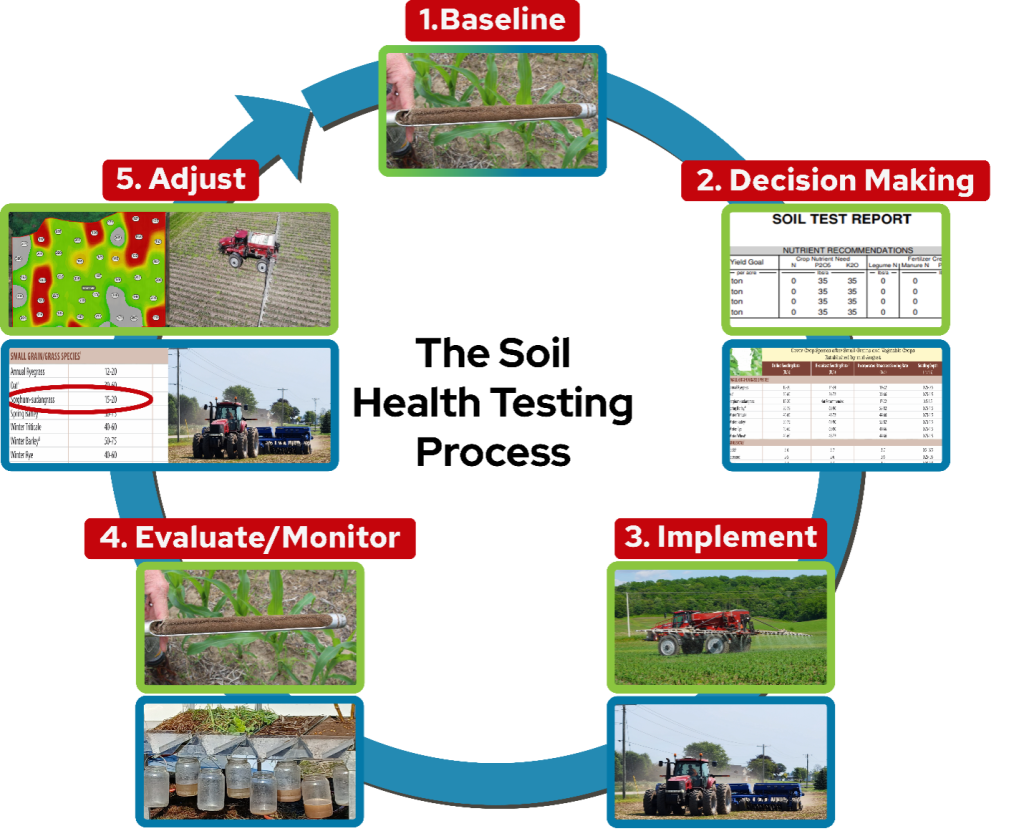
Introduction to Soil Health Testing
Farmers and agronomy professionals are interested in quantifying and monitoring changes in soil health on their acres. As such, several laboratories provide soil health testing services. There is, however, some debate among soil scientists and within the agricultural community as to what tests are best to use, and what the results of those tests really mean.
Laboratory tests for assessing soil health (including physical, biological, and chemical tests) are abundant1, so it is important to understand what these tests measure in the lab, what factors can influence their results, and what management practices can be used to improve the soil function that a specific test represents. This article aims to address these common questions for several soil health indicators. Subsequent articles in our series will further examine connections between these tests and management practices, with specific examples from Wisconsin.
This article will provide an overview for the following soil health indicators: Soil Organic Matter (SOM), Soil Organic Carbon (SOC), Potentially Mineralizable Carbon (MinC), Permanganate Oxidizable Carbon (POXC), Total Nitrogen (TN), Potentially Mineralizable Nitrogen (PMN), Autoclaved Citrate Extractable (ACE) Protein, and Aggregate Stability (AS).
Soil Organic Matter (SOM)
The amount of soil organic matter influences most soil functions. Organic matter is derived from several things including manure and/or compost applications, plant residues in varying stages of decomposition, and perhaps even more importantly, from the residues (dead bodies) of soil microorganisms, mostly bacteria and fungi. This new scientific understanding of soil organic matter is important because it challenges previous ideas, which proposed that most soil organic matter was made of complex “humic” materials that were formed in soil during the process of organic matter decomposition and were very difficult to decompose. Now, we understand that a major part of SOM is made up of simple organic compounds such as carbohydrates or proteins released during the decomposition of plants and microorganisms2. These simple compounds should be relatively easy to decompose, but they may be protected by binding with soil minerals and aggregates, potentially allowing them to persist for long periods of time (even decades to centuries2,3).
Soil organic matter has long been the most common soil test that has been conducted to monitor soil health and function4. Soil organic matter is a widely available, affordable, and easy-to-determine soil test. Most commonly, SOM is determined by a “loss-on-ignition” method, where soil samples are heated (typically660–750° F) for 2 hours to burn off the organic matter. The reduction in mass of a soil sample following the heating period mostly represents the SOM content, which is reported as a percentage of the initial soil mass (e.g. 3.0%). However, some soil minerals contain a small amount of water that is also lost during heating, so SOM measurements should generally be interpreted as “approximate” values5.
Soil Organic Carbon (SOC)
Soil organic carbon is primarily made up of organic material in the soil that is not living, although a small component of SOC is made up of living organisms. Soil organic carbon is highly related to SOM content, making up about 50% of the total SOM5. Soil organic carbon represents the balance of C inputs and outputs of the soil over time and is the main energy (“food”) source for soil microorganisms. Generally, total carbon is determined by combusting soil at a high temperature (often > 1800 °F) using an instrument known as an elemental analyzer, thereby converting all of the carbon to carbon dioxide (CO2), which is measured directly. In soils with pH < 7, total carbon is generally equivalent to organic carbon. Soils with higher pH values (especially when pH is > 7.8) may contain inorganic carbon, largely in the form of carbonate minerals. In these soils, carbonates can either be removed with acid prior to measurement of total carbon, or they can be measured separately. Thus, SOC is the difference between total carbon and inorganic carbon, calculated as follows:
SOC = Total Carbon – Inorganic Carbon
SOC is expressed as a percentage of total soil mass (i.e. % SOC, often referred to as SOC concentration). It is critical to emphasize that total carbon is not the same as SOC in soils with carbonate, and many commercial labs do not provide the necessary analyses to distinguish these important carbon forms. Carbonate is not a food source for microbes, and in large quantities, it binds with nutrients (e.g. phosphorus and iron) and can interfere with their uptake by the crop. Lastly, total carbon and SOC measurements are typically more stable than other carbon-based soil health tests and are less impacted by when samples are collected.
In recent years, there has been interest in using SOC measurements to assess carbon storage (the amount of SOC in the soil) and sequestration (the gain or loss of SOC over time) for carbon credit programs. In this case, bulk density (the mass of soil per unit volume) must also be measured. Then, the SOC stock, which is defined as the mass of SOC per unit area of soil, can be calculated as:
SOC Stock = SOC concentration x Soil bulk density x Sampling depth
Measuring changes or differences in SOC stocks is technically challenging, as other factors such as soil compaction or expansion must also be considered6. While SOC concentration measurements are a very useful soil health indicator, by themselves they cannot be used to infer the total amount of SOC in a field or the absolute change in SOC over time. Also note that sampling depth for SOC stock determinations is typically deeper than that for routine soil health evaluation.
Potentially Mineralizable Carbon (MinC)
Mineralizable carbon, also known as carbon respiration or the CO2 burst test, is broadly defined as the amount of SOC that is converted (“mineralized”) to CO2 by soil microbial communities under specific laboratory conditions including time, temperature, and moisture7. Mineralization refers to the conversion of carbon from an organic form to an inorganic form, and despite the name, it does not imply that a “mineral” has been formed.
MinC is typically measured by sealing a defined volume of dry soil in a jar, adding water to “wake up” the microbial community, and measuring the CO2 produced by soil microbes after a period of time (usually 1-4 days). Generally speaking, MinC is an indicator of soil microbial abundance (how much microbial biomass is present), food supply (how much C is available to eat), and activity (how much work are they doing).
It is worth noting that methods for determining MinC may differ between labs. For example, labs may vary in how samples were dried before measurement, how long the soil is sealed, the moisture and temperature of the soil, and how water is applied to the soil prior to the test (e.g., re-wetting by adding water to the top of the sample vs. from the bottom). These sources of analytical variability can affect test results and interpretation, especially if comparing results from different labs.
Also, MinC is sometimes reported in units that are not comparable among labs without subsequent calculations. For example, MinC is often expressed using units of CO2 in ppm, which is not directly interpretable unless we know the volume of the container that was used for incubating the sample, the air temperature, and the mass of soil that was used. A better choice would be to express MinC as the mass of CO2 released per mass of soil incubated. In general, most studies use an incubation temperature of 77°F (25°C). Warmer temperatures will increase MinC values, while cooler temperatures will lead to lower values. Again, it is currently not clear what the standardized methodology should be.
Permanganate Oxidizable Carbon (POXC)
Permanganate Oxidizable Carbon is a reactive fraction of SOC (typically several percent of total SOC8) that is thought to be related to biological decomposition activity9,10,11. POXC is measured by combining soil with a dilute solution of potassium permanganate, which reacts with certain carbon compounds, thereby consuming the permanganate and changing the color of the solution. This color change can be measured and expressed as the amount of carbon that reacted with permanganate.
Being a “subset” of total SOC, it is no surprise that POXC and SOC are positively related (i.e., greater POXC is generally associated with greater SOC). Although POXC was previously thought to represent C that was most readily available to microbes, current evidence suggests that POXC is mostly measuring “polyphenolic compounds” that accumulate during the process of organic matter decomposition11. Thus, POXC might be telling us something about the amount of residue available for decomposition, and how much decomposition has occurred, although the exact details remain unclear.
Total Nitrogen (TN)
Total nitrogen is the measure of all forms of N in soils, including organic N (e.g., N in soil organic matter and crop residues) in addition to inorganic N (e.g. ammonium and nitrate). Organic N makes up the largest fraction of TN, and often is not readily plant-available, whereas inorganic N makes up a small amount of TN but is immediately plant-available. Intriguingly, some crops are able to take up and use some soluble organic N compounds–an active area of research12.
Similar to total carbon, total N is typically measured by dry combustion of a small sample of soil at high temperature on an elemental analyzer, and total carbon and total nitrogen can often be measured simultaneously. Total N content of soils, and of plant and microbial residues, is an important factor when thinking about N cycling in agricultural systems. In general, soils with greater total N content will have greater N supply for plants, which we discuss next.
Potentially Mineralizable Nitrogen (PMN)
Potentially Mineralizable Nitrogen is the portion of soil organic N that has been converted to plant available forms (i.e. inorganic or mineral N, mostly ammonium or nitrate) under specific laboratory-controlled temperature and moisture conditions within a specific period of time. This fraction of N primarily comes from the decomposition of microbial biomass and plant/animal residues and is considered to be a readily available portion of soil organic N.
Different methods have been used to measure PMN, and results from these different methods are generally not comparable. One common method is the waterlogged (anaerobic) seven-day incubation, where soil samples are immersed in water and heated to 104 °F to stimulate microbial activity, and the increase in ammonium during the incubation is reported as PMN13. Alternatively, soils can be incubated under moist (aerobic, non-waterlogged) conditions for longer time periods, typically weeks to months, and the change in inorganic nitrogen (ammonium + nitrate) over time is reported as PMN. Regardless of the particular method, PMN is a relative indicator of a soil’s ability to mineralize N and is an indirect estimate of N that could be made available from organic-N throughout the growing season.
Autoclaved Citrate Extractable (ACE) Protein
Autoclaved citrate extractable (ACE) protein is a SH indicator that measures the amount of protein-like substances in SOM, which are a major form of soil N. ACE protein has been defined as the primary mineralizable (i.e. available to soil microbial communities) pool of organic soil N14. This pool of N, once processed by microbes, would result in plant-available N being released into soil (i.e., mineralized), making it available to plants.
ACE protein is measured by extracting proteins from soil using a citrate solution under high temperature and pressure. The extracted protein is then quantified using a colorimetric test (how much light a colored solution absorbs) to estimate the amount of soil protein in the sample.
Aggregate Stability (AS)
Aggregate stability is a physical soil property which indicates the ability of soil aggregates to resist disintegration when forces associated with tillage and water/wind erosion are applied. Aggregate stability is influenced heavily by SOM, SOC, and soil biological activity. Byproducts of soil microbial functions and plant root activity (i.e., exudates) act as “glues” that bind soil particles together to form aggregates.
Traditionally, aggregate stability and/or size distribution has been measured by passing soil through one or a series of sieves with specific mesh sizes, either dry or submerged under water, and measuring the amount of soil that was retained on the sieve15. Recently, image analysis has been successfully used to quantify soil aggregation16 and this method has been incorporated into the SLAKES smartphone app, enabling a quick and inexpensive measurement of relative aggregate stability. As with the other soil health metrics, different AS methods are not directly comparable and results can be different between labs, although results may be loosely correlated17.
Management and Soil Health Tests
Adoption, and long-term implementation, of specific management practices can impact soil health tests, providing a way to measure and track improvements in how the soil functions. Figure 1 below shows the relative impact that different management practices have on the eight soil health tests discussed in this article. The practices displayed in Figure 1 are defined as follows: Crop Count: Comparison of monoculture treatments to those with more than one cash crop in the rotation; Reduced Tillage: Comparison of treatments with lower standard tillage intensity ratings (STIR, NRCS – therefore reduced tillage intensity) to those with higher STIR values (more intensive tillage); Cover Crops: Comparison of treatments with at least one year of cover crops in the cropping system versus those without cover crops; Organic Amendments: Comparison of treatments that received organic inputs (biosolids, compost, or manure) to those that did not; and Residue Retention: Comparison of treatments that were managed identically other than the amount of biomass removed following grain harvest.
An example for how to interpret Figure 1: Soil organic carbon (SOC) can often be increased following long-term use of: A) Reduced tillage (frequency and intensity); B) Cover crops; C) Organic amendments (i.e. manure or compost); and D) Crop residue retention (Fig. 1).
The data summarized in Figure 1 comes from the North American Project to Evaluate Soil Health Measurements (NAPESHM) – a large summary of research trials at over 120 long-term agricultural research sites across North America18. While the soil health indicators discussed in this article are all sensitive to changes in soil management, they may respond at different timescales. For example, SOM responds more slowly to changes in management compared to other soil health indicators, like MinC and POXC, which sometimes respond more rapidly and may act as proxies for longer-term expected changes in SOM. Similarly, changes in TN resulting from changes in soil and crop management are very slow compared to indicators like PMN and ACE-protein. In general, the largest benefit to soil health occurs when there is an increase in carbon additions to soil.
Figure 1. Long-Term Management Practice Impacts on Soil Health Tests (NAPESHM)
| Soil Health Tests | Crop Count | Reduced Tillage | Cover Crops | Organic Amend. | Residue Retention |
|---|---|---|---|---|---|
| Organic Matter (SOM) | Neutral | Neutral | Weak | Weak | Weak |
| Organic Carbon (SOC) | Neutral | Weak | Weak | Weak | Weak |
| Mineralizable Carbon (MinC) | Neutral | Weak | Strong | Weak | Strong |
| Permanganate Oxidizable Carbon (POXC) | Neutral | Neutral | Weak | Weak | Weak |
| Total Nitrogen (TN) | Neutral | Weak | Weak | Weak | Weak |
| Potentially Mineralizable Nitrogen (PMN) | Neutral | Neutral | Weak | Weak | Weak |
| Autoclaved Citrate Extractable (ACE) Protein | Neutral | Neutral | Weak | Weak | Weak |
| Aggregate Stability (AS) | Neutral | Weak | Strong | Neutral | Weak |
Weak = weak positive impact
Strong = strong positive impact
Figure 1. Relative impact of various, long-term management practices on soil health tests from across North America (Adapted from Bagnall et al., 2023). See section above for additional details.
*It is important to emphasize that data from NAPESHM18 were derived from replicated, long-term, comparative field trials. What does this mean? For example, to evaluate the impact of long-term cover crop use on soil health tests, soil health test values from replicated areas within the field that did not have cover crops were compared to those from replicated areas of the field that did have cover crops. The average difference in soil health test values between cover crop and no-cover crop was then recorded. This type of research is very different from that which uses random sampling across farms or research stations with varying durations of practice adoption and without the comparative aspect (i.e. with vs. without cover crops). The data shown in Figure 1 represent broad-scale responses to management changes, and there is a need for the development of state and/or soil-specific soil health test recommendations and interpretations. This information is at an early stage of development for Wisconsin.
Concluding Thoughts
The list of soil health tests presented in this article is by no means exhaustive; however, these indicators are commonly evaluated in research studies that are conducted in both on-farm and research station settings. Meaningful, science-based, assessments of field and soil management impacts on these soil health indicators are widely available and data suggests that these indicators (among others) can act as tools that inform us about the state of our soils’ health and ability to function. Additionally, they serve as a tool for continued monitoring and evaluation of our fields over time as we make changes to our management.
Updated: July 9, 2025
Reviewed by: Francisco Arriaga and Abby Augarten
Related Articles

Soil Health Lab, Sampling, and Test Selection Considerations
This article provides guidance related to the importance of using the same lab to assess changes in soil health over time, in addition to the importance of sampling at a consistent time of year and depth when collecting samples for soil health evaluation.
Soil Health in Wisconsin: Characteristics of Healthy Soil
This article highlights the characteristics of healthy soil and describes how these characteristics, and soil health generally, can contribute to sustainable crop production and indirectly impact society beyond the farm field.
References
- Stewart et al., 2018. What we talk about when we talk about soil health. Agricultural & Environmental Letters. https://doi.org/10.2134/ael2018.06.0033
- Lehmann and Kleber, 2015. The contentious nature of soil organic matter. Nature. https://doi.org/10.1038/nature16069
- Waring et.al. 2020. From pools to flow: The PROMISE framework for new insights on soil carbon cycling in a changing world. Global Change Biology. https://doi.org/10.1111/gcb.15365
- Bünemann et al., 2018. Soil quality – A critical review. Soil Biology and Biochemistry. https://doi.org/10.1016/j.soilbio.2018.01.030
- Pribyl, 2010.A critical review of the conventional SOC to SOM conversion factor. Geoderma. https://doi.org/10.1016/j.geoderma.2010.02.003
- Dietz et al., 2024. Soil carbon maintained by perennial grasslands over 30 years but lost in field crop systems in a temperate Mollisol. Communications Earth & Environment. https://doi.org/10.1038/s43247-024-01500-w
- Diederich et al., 2019. Increasing labile soil carbon and nitrogen fractions require a change in system, rather than practice. Soil Science Society of America Journal. https://doi.org/10.2136/sssaj2018.11.0458
- Liptzin et al., 2022. An evaluation of carbon indicators of soil health in long-term agricultural experiments. Soil Biology and Biochemistry. https://doi.org/10.1016/j.soilbio.2022.108708
- Culman et al., 2012. Permanganate oxidizable carbon reflects a processed soil fraction that is sensitive to management. Soil Science Society of America Journal. https://doi.org/10.2136/sssaj2011.0286
- Weil et al., 2003. Estimating active carbon for soil quality assessment: A simplified method for laboratory and field use. American Journal of Alternative Agriculture. https://doi.org/10.1079/AJAA2003003
- Christy et al. 2023. A mechanistic inquiry into the applicability of permanganate oxidizable carbon as a soil health indicator. Soil Science Society of America Journal. https://acsess.onlinelibrary.wiley.com/doi/full/10.1002/saj2.20569
- Bundy and Meisinger, 1994. Nitrogen Availability Indices. In Methods of Soil Analysis. https://doi.org/10.2136/sssabookser5.2.c41
- Farzadfar et al. 2021. Soil organic nitrogen: an overlooked but potentially significant contribution to crop nutrition. Plant and Soil. https://doi.org/10.1007/s11104-021-04860-w
- Hurisso and Culman, 2021. Is Autoclaved Citrate-Extractable (ACE) protein a viable indicator of soil nitrogen availability? In Soil Health Series. https://doi.org/10.1002/9780891189831.ch10
- Kemper and Rosenau, 1986. Aggregate Stability and Size Distribution. In Methods of Soil Analysis. https://doi.org/10.2136/sssabookser5.1.2ed.c17
- Fajardo et al. 2016. Soil slaking assessment using image recognition. Soil and Tillage Research. https://doi.org/10.1016/j.still.2016.05.018
- Rieke et al. 2022. Evaluation of aggregate stability methods for soil health. Geoderma. https://doi.org/10.1016/j.geoderma.2022.116156
- Bagnall et al., 2023. A minimum suite of soil health indicators for North American agriculture. Soil Security. https://doi.org/10.1016/j.soisec.2023.100084