Introduction
Preventing mycotoxin contamination in corn silage requires a multi-pronged approach, including field management, proper harvesting, ensiling techniques, and strategic feeding practices. Regular testing and proactive adjustments can help maintain silage quality and safeguard animal health and performance. By following these guidelines, farmers can minimize the risks posed by mycotoxins and ensure a healthier, more productive livestock feed supply.
For key points of this article, click here.
What are mycotoxins?
Mycotoxins are toxic compounds that fungal organisms can produce. These toxic compounds are considered secondary metabolites, meaning they are not produced during primary metabolism. Our current understanding is that these compounds are produced by fungi in response to stress and/or competition by other fungi. Many different fungi produce mycotoxins with thousands of these toxic compounds known. When mycotoxins are consumed by animals they can cause mycotoxicosis, with symptoms varying widely depending on the toxin(s) involved and the animal species affected. Mycotoxins can be produced in the corn field or in storage. The environment and any damage to the plants can also contribute to the presence of these toxic compounds. Mycotoxins can also depress the health and performance of animals in a negative synergistic way, where multiple mycotoxins each affect the animals in different ways.
Where do mycotoxins like deoxynivalenol (DON) come from?
Gibberella ear rot is caused by Fusarium graminearum (a.k.a. Gibberella zea). The same pathogen can cause Gibberella crown and stalk rot in corn. We have observed both diseases on silage corn in Wisconsin in recent years. Our growing environment in the Great Lakes region makes a perfect place for this pathogen to cause these diseases. In addition to the plant tissue damage that the pathogen can cause, the fungus can also produce various mycotoxins, most notably deoxynivalenol (DON or Vomitoxin). In paired, seperated plant part experiments, we have found that DON can accumulate in both the stalk and ear portions of the plant, and that these two phases of accumulation are not linked to each other.
The fungus can infect these parts separately at different times during the season and the subsequent accumulation of DON can happen differentially in the stalks vs. the ears. This is partially why you can go out to the field and scout for ear rot and not see a lot of infection (moldy ears), but still have high DON levels at chopping time. Some of that DON is likely accumulating in the stalks. This is an important take home point, as mycotoxin contamination is a substantial health and performance issue which often can’t be fully mitigated by nutritionists.
How can deoxynivalenol be managed in the field?
Many factors influence the presence or absence of DON in the field. The fungi that can cause DON can survive in Wisconsin on corn residue. While managing in-field residue is a possible way to reduce inoculum levels in a field, there is still high potential that the fungi can blow in on weather systems. There is a lot of corn in the Midwest, and the fungus is ubiquitous, thus we would not want farmers to choose tillage over soil conservation.
Hybrid type and plant resistance is a key mitigation opportunity. We do tend to see brown mid-rib (BMR) hybrids having higher levels of DON accumulation when compared to conventional or multi-purpose hybrids. If a farmer is struggling over multiple seasons in a particular field with DON, and they are growing BMR hybrids; simply moving away from BMR hybrids might help reduce the risk of DON accumulation. Managing plant populations to reduce in-field humidity levels and reducing inter-plant competition for nutrients is important to improve plant health and reduce the risk for mycotoxin accumulation.
Foliar fungicides applied in-season can be part of the management plan for DON. However, be aware that in Wisconsin, the application of fungicides has been inconsistent in reducing DON concentrations. Many factors likely influence success, but some of the issue is that the window of opportunity to apply fungicides is narrow beginning at white silk and only extending 5-7 days after white silk appearance. It has been demonstrated that the loss in fungicide efficacy can be considerable when these products are applied outside of this window. In addition, if the fungal infection in corn ears is due to husk and silk damage caused by ear feeding insects, fungicide applications may not be very beneficial. In those cases the use of Bt protection traits in silage hybrids or the use of certain pyrethroid based insecticides may help. One benefit of fungicides in some years is that they can also control foliar disease problems. Foliar diseases like tar spot lead to a reduction in overall silage quality, but they can also force the plant to scavenge carbohydrates in the stalk which can result in standability issues and lodging. These diseases can also accelerate crop dry down, narrowing the harvest interval, making harvesting at optimal moisture difficult. If silage is harvested at sub-optimal moisture, silage packing, density and fermentation are challenged. Subsequently, spoilage by mold, yeast and other undesirable aerobic organisms are more likely. This can indirectly lead to an increase in mycotoxins and “mold” issues from these aerobic fungi. Spoilage yeast can disrupt rumen metabolism and exacerbate the feeding issues.
Focus your attention on making good silage at chopping time. Harvesting at the optimum moisture content for your storage system is of high priority. If foliar diseases are a concern, consider chopping a bit earlier to reduce continued mycotoxin accumulation, promote fermentation, and limit dry-matter yield losses; while subsequently focusing on good bunker management. If moisture at chopping is not prioritized, then subsequent storage issues as described above will take hold and will endure for the duration the silage is fed out.
Finally, test for DON as part of your regular silage analysis program. Frequent testing with a new crop can give way to less frequent or responsive testing later on, depending on the initial levels found in silage. Also test for other anti-nutritional factors such as spoilage mold, yeast and bacteria as these feed hygiene factors will act synergistically, and negatively affect herd health and performance. Understand what you are dealing with in terms of DON and other feed contaminant concentrations so you can make informed decisions with your rations.
How does deoxynivalenol increase in stored corn silage?
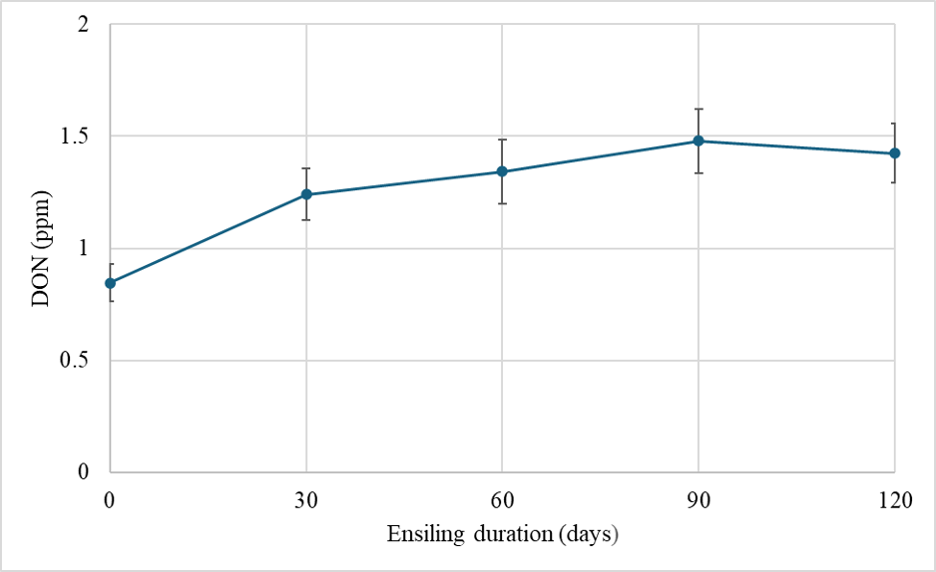
We have also been following the fate of DON in silage harvested and chopped from a brown midrib (BMR) hybrid and a dual-purpose hybrid that were grown in the field and treated with fungicides at white silk (R1). We chopped the plants in each plot and then used mini-silos to conduct a time-course experiment tracking DON levels in the mini-silos. In all cases we saw DON levels generally increasing in the first 30 days after chopping (Figure 1). They then leveled off and became stable at 60, 90, and 120 days after chopping. This observation is not well recognized by the dairy industry, however is important to grasp. Some of this increase could be due to oxygen still in the system during the first 30 days after chopping. DON-producing fungi are aerobic and continue to consume some of the minute levels of oxygen still in the system, thereby still producing DON. However, this likely only explains some of the DON levels we detected. Proper packing and sealing silos with oxygen-barrier plastic films can reduce oxygen penetration, fungi infestation, and spoilage of silage.
Figure 1. DON concentration across ensiling time from the day of chopping until 120 days after chopping and ensiling
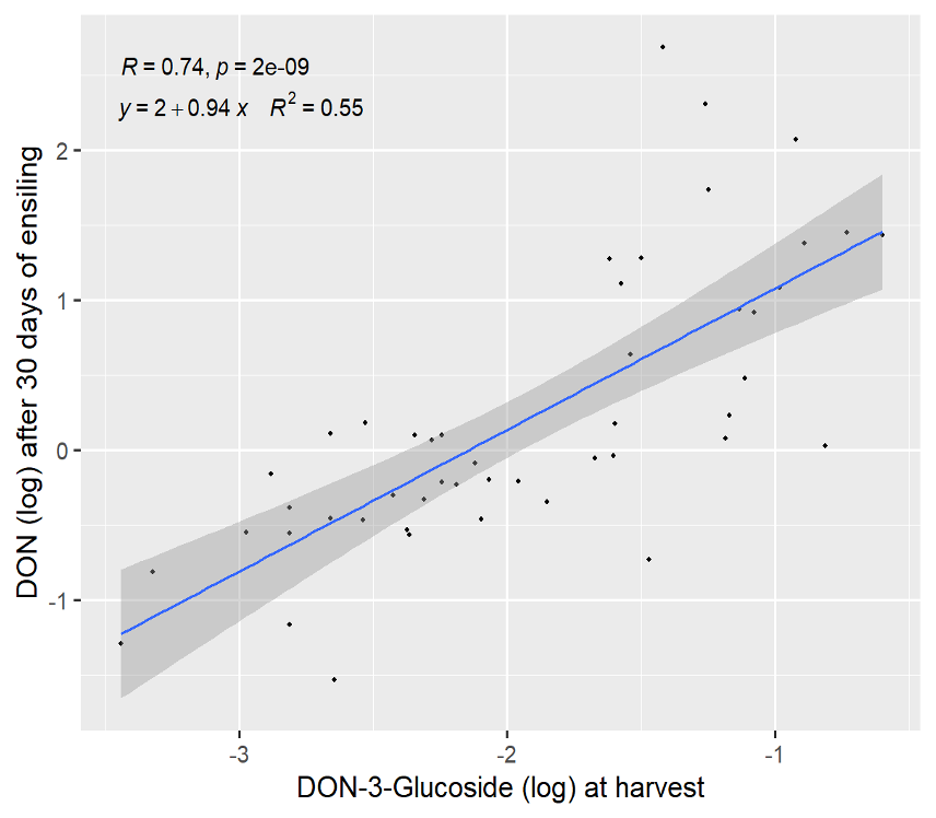
There are also “masked” or conjugated forms of DON that are not detectable in routine DON analyses. One such conjugate is DON-3-glucoside (D3G). D3G can be produced by fungi or during a plant’s attempt to protect itself from the toxicity of DON. Either way, D3G is not detectable in routine DON tests but is important to consider and can only be measured with a specific D3G test. We investigated our samples further during the first 30 days of ensiling and found that the level of D3G at harvest explained a significant level of the DON increase uncovered in samples after 30 days of ensiling. This is to say that D3G present at harvest is likely metabolized in the first 30 days of ensiling, thereby releasing DON and resulting in higher DON levels 30-days later (Figure 2).
Again, this relationship only partially explains why DON increases in silage during the first 30 days of storage. The full explanation is likely due to both metabolization of D3G and continued fungal respiration leading to an increase in DON at feed out compared to when it was packed in the bunker. If your farm measures DON at harvest, consider this a baseline and results may potentially increase over time in the silo.
Figure 2. Relationship of DON-3-Glucoside (log) at harvest and DON concentration after 30 days of ensiling.
How can deoxynivalenol and other mycotoxins be managed going into the bunker?
You need to balance foliar disease management with Gibberella/DON management when making high-quality silage in Wisconsin. Diseases like tar spot are the new normal resulting in quick and early drying of the crop. As you prepare to harvest, it is a good idea to get out in the field and see how bad the foliar disease is and how much ear rot you are seeing. You will want to prioritize harvesting the diseased fields first. In fields heavily contaminated with tar spot monitor moisture carefully and chop to optimize moisture. Harvesting a bit sooner than later is helpful in these challenging situations.
Concentrate on good bunker hygiene and spend added time packing the feed when disease hits and moisture is less than ideal, focusing on getting as much oxygen out of the system as you can. If a corn crop becomes too dry to make good silage, you might consider harvesting it for snaplage or high-moisture grain to try to circumvent bigger issues that could arise at feed out associated with making suboptimal silage.
Finally, it is important to test for DON and other feed hygiene contaminants frequently and understand what you are dealing with. You want to start with the lowest levels of DON coming from the field that you can. DON will likely increase in the bunker no matter how well you pack it. Thus, starting with the lowest levels at harvest will help keep final levels of DON below critical thresholds. Moving forward, mycotoxin testing in corn should include not only DON but also consider DON conjugates that can be metabolized back to DON and increase the final DON concentration during ensiling.
How can deoxynivalenol and other mycotoxins be managed at feedout?
Mycotoxins present in silage will be a prevailing issue, which will not go away. These concerns intensify in years when environmental conditions during the forage growing season enable fungal infection and growth. Mycotoxin concentration should be tested at feed-out and understood. Use this information to make decisions about of the contaminated silage might be blended with other cleaner feeds to minimize the total mixed ration mycotoxin load. Guidelines for mycotoxin limits in total mixed rations are available here.
Although mycotoxins can never be completely broken down or denatured, a variety of feed supplements are available to partially mitigate mycotoxin impact on dairy herd health, digestion and performance. These include mycotoxin binders, yeast-based and probiotic supplements to aid rumen health and digestion, immune system boosting supplements and other helpful feed technologies. There is no single solution to manage mycotoxin contamination, a multi-faceted nutrition program will be needed to manage contaminated silage.
Avoid additional spoilage issues at feed out by minimizing the silage exposure to oxygen to the extent possible. Deface or remove silage at feed mixing, keeping an even silo face. Do not perform this procedure more than one or two hours prior to feeding, if possible. If spoilage yeast are growing rampantly and silage is heating, consider mixing a preservative with the silage to stabilize the silage prior to mixing in with other feeds in the ration. If silage stability and heating has been a common issue consider using a Lactobacillus buchneri-based silage inoculant.
When feeding out of bunkers or piles, avoid cutting back more than 2 or 3 days worth of plastic and line the plastic with multiple tire layers or gravel bags to prevent air and oxygen penetrating underneath the plastic. If feeding out of bags, monitor for bird or rodent damage in the bags and fix holes in the plastic promptly with tape.
Other Resources
I See Dead Plants Podcast, Bunker of Doom: The Mycotoxin Threat in Corn Silage Part 1 – https://podcasts.apple.com/us/podcast/s3-e44-bunker-of-doom-the-mycotoxin-threat-in/id1579753424?i=1000684088257
I See Dead Plants Podcast, Bunker of Doom: The Mycotoxin Threat in Corn Silage Part 2 – https://podcasts.apple.com/us/podcast/s3-e45-bunker-of-doom-the-mycotoxin-threat-in/id1579753424?i=1000685010603
Four Ways to Get Ahead of Ear Rots and Mycotoxins – Crop Protection Network
Corn Grain and Silage Sampling and Mycotoxin Testing – Crop Protection Network
References
Chibuogwu, M. O., Groves, C.L., Mueller, B., and Smith, D.L. 2024. Effects of fungicide application and corn hybrid class on the presence of Fusarium graminearum and the concentration of deoxynivalenol in ear and stalk parts of corn (Zea mays) used for silage. Plant Disease. https://doi.org/10.1094/PDIS-12-23-2662-RE
Chibuogwu, M. O., Reed, H., Groves, C. L., Mueller, B., Barrett-Wilt, G., Webster, R.W., Goeser, J., and Smith, D. 2024. Influence of hybrid class and ensiling duration on deoxynivalenol accumulation and its derivative deoxynivalenol-3-glucoside while ensiling corn for silage. Plant Disease. https://doi.org/10.1094/PDIS-06-24-1166-RE
Chibuogwu, M. O., Mueller, B., Groves, C. L., and Smith, D. 2023. Impact of fungicides on dual-purpose and brown midrib Zea mays hybrids used for silage in Wisconsin. Plant Health Prog. 24:462–467. https://doi.org/10.1094/PHP-04-23-0036-RS
Reed, H., Mueller, B., Groves, C., and Smith, D.L. 2022. Presence and correlation of Fusarium graminearum and deoxynivalenol accumulation in silage corn plant parts. Plant Disease. https://doi.org/10.1094/PDIS-03-21-0641-RE.
Reed, H., Mueller, B., Groves, C., and Smith, D.L. 2021. Impact of foliar fungicides on disease and silage quality of brown midrib (BMR) corn hybrids in Wisconsin. Plant Health Progress. https://doi.org/10.1094/PHP-02-21-0019-RS.
Updated: Feb. 5, 2025





